Seen boosting security as drugs move from factory to pharmacy
WASHINGTON — About seven out of ten Americans takes a prescription drug. Though most of us don’t think twice about the journey our medicine takes from factory to pharmacy, the drug supply chain is in trouble.
About 10% of the world’s drugs are tainted or unsafe, and the counterfeit drug market is now a $217-billion industry. Because drugs are manufactured all over the globe, products change hands dozens of times before ending up on pharmacy shelves. That creates plenty of opportunity for fake, expired or contaminated drugs to infect an increasingly chaotic supply chain.
The FDA and other regulatory bodies have tried to fix this problem by asking companies to employ audit trails and barcode-based tracking systems, all with limited success. But according to healthcare experts gathered at the 2018 Blockchain Health Summit here last week, a new technology called blockchain is poised to fix the fractured drug supply chain problem.
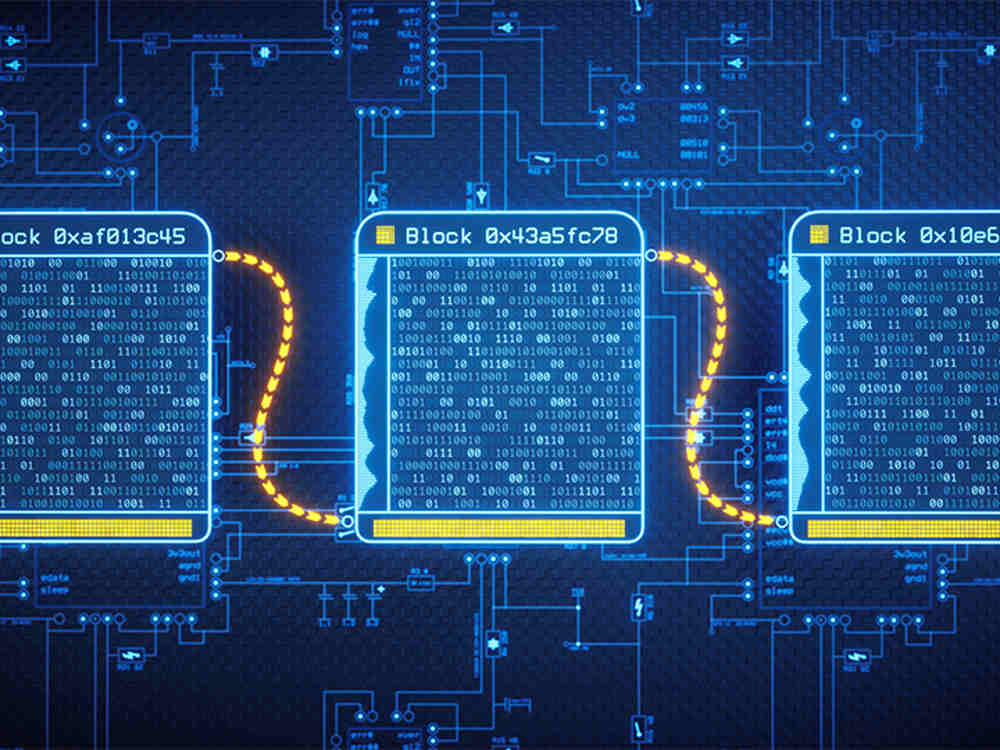
Blockchain rose to prominence as the backbone of the cryptocurrency Bitcoin, but it is now finding a home in healthcare. In essence, it’s a turbo-charged accounting system that encrypts and stores transactional data across multiple computers, making it virtually tamper-proof. Better yet, blockchain provides a coherent record for all participants to see — akin to a shared Google Drive document.
Within the highly regulated drug industry, blockchain could bring regulators, manufacturers, distributors and pharmacies on the same page and provide a verifiable record of a drug’s journey across the globe. Eventually, the federal government could use blockchain to prepare for drug shortages, predicted Jim Nasr, vice president for technology and innovation at Certara’s Synchrogenix unit.
Blockchain exploded onto the healthcare scene about 5 years ago, just as Congress passed the the 2013 Drug Supply Chain Security Act (DSCSA) — a new regulation designed to fix the broken supply chain.
DSCSA lays out a set of requirements around tracking and tracing drug products over a 10-year period. That means by 2023, pharma companies will be required to have an “interoperable, electronic tracing of product at the package level,” according to the regulation.
Since then, a handful of startups with names like Cryptowerk, Ambrosus, FarmaTrust, Spiritus and LinkLab have sprung up to offer blockchain platforms for supply-chain management. Their clients are pharmaceutical companies, raw ingredients suppliers, wholesalers and pharmacies.
In the U.S., blockchain is taking off because of the top-down regulatory pressures imposed by DSCSA. But in some developing countries, where counterfeit products may represent up to 30% of the drug supply, blockchain is being adopted as a way to combat rampant fraud.
Source/More: Blockchain Holds Promise for Thwarting Drug Counterfeiters | Medpage Today